Digital health intellectual property rights in India
With global efficient health delivery requirements and the popularity of the digital health sector progressively rising, the question of whether India’s intellectual property (IP) laws are sufficiently matured to cause a digital health revolution becomes a very important one. The pace at which the Indian healthcare sector has been growing is quite healthy. It has been forecasted to grow from currently being a $100 billion industry to a $280 billion industry by the year 2020. With such expected 280% growth for the industry 3 years, this article takes a look at the nation’s IP laws and whether they are matured enough to trigger such a digital health revolution.
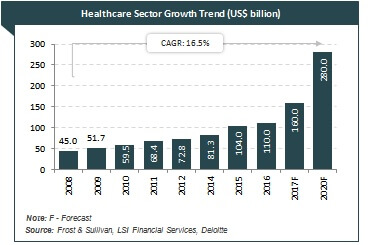
Image courtesy of IBEF
What are Intellectual Property Rights?
IP rights are the rights of intellectual property owners which are legally protected to apply monopoly control over the exploitation of their IP rights, normally for commercial benefits. IPRs enable inventors to prevent others from exploiting their IPs for particular periods of time according to the IP type. IPRs usually involve the following each frequently called industrial property:
- Patents
- Industrial designs
- Geographical indication of goods
- Protecting undisclosed information like trade secrets
- Trademarks
- Copyrights
- Integrated circuits
With the nation’s authorities using fresh policies like the 2016 National Intellectual Property Rights Policy to push for innovations, fresh incentives like those that come through the ‘Startup India’ policy now available for startups, and various players currently debuting in the healthcare market, the digital health sector has been positively impacted.
The nation’s newest IP policies have brought several promising players into the digital healthcare market, which has, in turn, attracted large-scale investor interest that has seen digital startups getting enough funding at varying stages to improve their business.
IPRs help digital health researchers to develop and safeguard knowledge for creating processes and products which can be commercialized. This allows inventors to concentrate on undertaking commercially and socially relevant research since IPRs give them ‘legal rights’ that could lead to monetary rewards over their efforts. It is what has led to the latest boom in mHealth and eHealth innovations that have given birth to varying digital health applications and devices.
Several organizations and key stakeholders are involved in the Indian IPR domain. The domain features more than 300 stakeholders including individuals and several organizations. These organizations which formulate policies to play the role of creating IPR awareness, using varying relevant measures to stimulate the creation and growth of IP, strengthening and modernizing IP administration, catalyzing the commercialization of inventors’ IP rights among others, include Creative India,Innovative India, DIPP (Department of Industrial Policy & Promotion), Ministry of Commerce, CIPAM (Cell for IPR Promotion & Management), WIPO (World Intellectual Property Organization), and ICMR (Indian Council of Medical Research).
| Recommended for you | |
| India in process of setting up National Digital Health Authority | |
| Can startups fix the Indian healthcare sector? | |
| Changing regulations can kill your digital health startup |
Major IP Policies in India
Indian IP policies which are mostly implemented through the ICMR are all geared towards articulating the nation’s need to support all creative activity those of the digital health sector included, inspiring open ideas dissemination, and recognizing and rewarding innovative inventors. Varying IP-related laws, regulations, regimes, edicts, and international conventions and treaties India is committed to beginning from The Indian Penal Code of 1860, through the Convention and Statute on Freedom and Transit of October 31, 1922, to the National Intellectual Property Rights of 2016 are all geared towards offering clarity on the general focus of organizations’ R&D objectives, helping innovative digital health inventors to file patents, and helping the industry in approaching the agency for the commercialization of valuable inventions.
The IP policies offer techno-legal and several other expert support and assistance to digital health scientists to file for patents both within the nation and abroad.
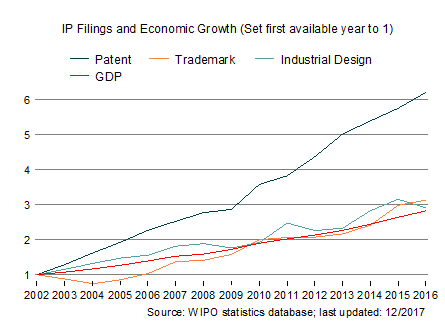
Image courtesy of wipo.int
The created IP is therefore exploited to bring out processes and products for public health with the main focus being on enhancing digital health R&D and affordable digital healthcare.
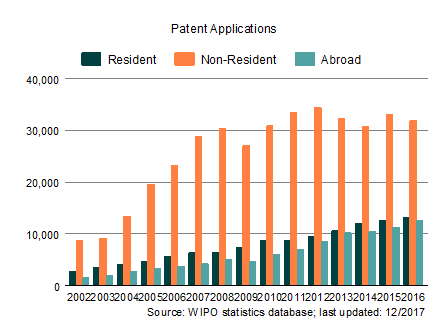
Image courtesy of wipo.int
An application for a patent is to be filed soonest after completing an invention without public disclosure as any public disclosure of any invention could make its patent filing null and void. Applications could also be filed with provisional specification showing the invention’s nature essence without elaborate patent claims. This helps in prioritizing of such inventions.
The full term of an Indian patent is 20 years from when the application was filed and this applies to every type of invention. Information needed for an application include invention’s title, name, address, and nationality of the patent applicant(s), description, claims, and an abstract of the invention to be patented.
The post-TRIPS Indian IP regime has seen much higher R&D investments, with a detailed econometric exercise showing a move towards a more powerful IP regime causing a greater R&D activity thrust in the sector, and a significant rise in domestic and international patenting by domestic companies.
Infringing any type of IP is a cognizable offence in India. The offender could be liable to a temporary or permanent injunction, or imprisonment that could be between 6 months to 3 years with a fine of a minimum of 50,000 rupees which could extend up to 2 lakh rupees.
The other vital aspects of IP are technology transfer which involves the transfer of an inventor’s IP or other rights to an entity to utilize in commercializing a new invention, (this is typically done after using either copyright of patent to protect the IPR), and licensing which is a legally binding agreement between a licensor (the owner of an invention) in which he grants the licensee the rights to produce, utilize, or/and sell the invention within the license agreement’s framework.
Do current IPR policies impact the growth of digital startups?
India’s current IPR policies and framework are well established and stable from a legal, administrative, and judicial point of view. They are also completely compliant with all agreements concerning trade-related aspects of IPRs. This is not unconnected with all the steps that nation has taken to adjust and improve on all aspects of its IP regimes.
Take the TRIPS agreement for example, in principle, it integrates all forms of IPs and targets the firming and complementary protection standards, and provides for operative enforcement both at the national and international levels.
It addresses general GATT principles applicability and provisions of the international agreements on intellectual property (Part I). It also creates standards for scope, readiness, and usage (Part II), enforcement (Part III), acquisition as well as maintenance (Part IV) of IPRs. Additionally, it also addresses associated dispute avoidance and settlement mechanisms (Part V). Parts VI and VII of the agreement address formal provisions and deal with transitional, and institutional activities, respectively. Considering the fact that copying and infringing an IP is quite easier than undertaking biomedical research, the Indian authorities are using these IP policies and regimes to provide the much-needed protection for innovations and IT startups.
Nevertheless, the internet has significantly simplified copying of copyright material hence it is termed the world’s biggest copying machine. Although the Indian government has emplaced specific legislation to mitigate the various kinds of IPR infringement, this legislation is however not well equipped updated to handle modern and contemporary copyright violations.
Additionally, some basic challenges faced by the Indian IPR domain today include compulsory license, patent validity, issues in patent administration which include pendency, access to information challenges with respect to enforcement amongst others.
Other observed challenges include patent cost and cost of maintaining the rights as well as the potential cost arising from patent litigation.
Despite the foregoing, the Indian authorities have continually explored ways of ensuring IP security. Some of these efforts include the implementation of a makeover of the Indian healthcare sector which has yielded various benefits.The influx of varying international big players into the sector is among such benefits.
The final verdict
Healthcare delivery requirements and the progressive rise in the popularity of digital health make the effective and efficient implementation of IPRs very important in India. With major agencies and organizations like CIPAM and ICMR being set up by the authorities, and several policy amendments, and international treaties like the Doha treaty being implemented, current Indian IP policies are certainly enough.
Even though there is still room for improvement they are sufficient to provide protection for the rapidly growing healthcare R&D and innovations. This is clearly shown by all Indian IP fillings rising from 97,533 in 2002 to a whopping 317,681 in 2016. The amazing 326% increase in 14 years is a good indicator that current Indian IPR policies are completely protecting the rapidly increasing digital healthcare innovations.
What is just left for the authorities to do is to ensure that more efforts are geared towards ensuring that Indian IP policies meet international standards.
Such modifications and amendments to former IP laws and regimes will certainly show the world that India’s approach to a new IPR regime is to additionally prepare itself for global trade competition.
Image credit: www.dipp.nic.in