Why can’t startup founders afford to ignore digital health policy?
The worth of the global digital health market is rapidly increasing. In 2014, it was worth £23 billion and is expected to nearly double to £43 billion by the end of this year. Although currently the tiniest digital health market sub-category, mHealth apps appear to be the most promising market when it concerns growth. Their global market was predicted to grow by 49% annually from 2014 to the end of this year. Against this background, government regulations and policies have played their own role in the digital health sub-sector. Global governments are all implementing considerable changes to current regulatory structures and policies to support the sector. This article takes a look at such policies and how important they are to the digital health industry.
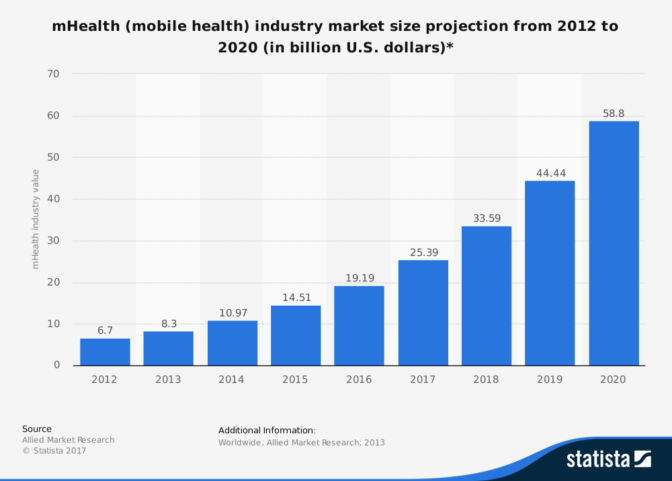
Image courtesy of Allied Market Research
What do we mean by government regulation or compliance in digital health?
Government regulations and policies for the digital health domain are complex sets of legal requirements that guide investors that want to establish medicine stores, start selling prescribed medications or digital health devices and apps online. The digital health sector is truly breaking out. More than $4.7 billion was poured into it in 2017 alone, and the market is expected to reach a whopping $536 billion by the year 2025 says TMR (Transparency Market Research).
Considering the seriousness of the digital health sector that’s attracting such massive investment and rapid growth, and the fact that it is an industry in which the life of so many people is at stake, it then becomes paramount that governments must develop regulations and policies that will govern the digital health sector.
These regulations and policies comprise guidelines for the operations of all stakeholders in the digital health industry. In India for example, The Clinical Establishments (Registration and Regulation) Act 2010, as well as the Clinical Establishments (Central Government), Rules 2012 were both mainly targeted at the digital health industry. Government regulations and policies are enforced to ensure that:
- Digital health innovations pass through a regulatory sieve before they are made commercially accessible.
- That research and innovations fulfil medical research-related terms under various global governments’ drugs and cosmetic policies and acts.
- That all medical testing and procedures laws and rules involved in medical products and drugs prescriptions are fully complied with.
- To guarantee a consistent great standard or safety, health, and protection for individuals using digital health innovations and research.
- To make sure that every notable scientific development that occurs within the digital health industry must conform to government directives.
- To promote innovation yet uphold the security aspect, support data privacy, and meet all safety requirements in accordance with the laws of any particular nation.
The startups are now to operate under varying regulations that include government policies that guide digital health mobile app providers, regulatory frameworks for applications, policies that guide digital health electronic data exchange, and those that enforce technical security standards.
All players in the digital health sector including investors, entrepreneurs, hospitals, and patients are impacted by these regulations.
These stakeholders benefit from the regulations as they help them to easily expand, enhance their legal confidence, boost their reputation and validate the establishments, and help them in streamlining their organizational processes.
These benefits certainly help digital health startups to grow and expand their outreach greatly. CCS Insight’s market research shows that 411 million smart wearable devices will be bought in 2020, which represents a worth of $34 billion in sales or a 143% rise compared to wearable devices’ sale for 2016. The regulatory framework cannot afford to ignore this kind of tremendous growth. This shows that although global authorities need to use regulations and policies to keep a close tab on the industry, there is also the need for a tradeoff between the policies and liberalization to enable the industry go at its own steady pace.
Why do policymakers play a critical role in digital health regulations?
Total investor funding in global digital health funding rose from $1.2 billion in 2010 to $11.5 billion in 2017. With a rise of about 960% in 7 years, the sector has played host to lots of newcomers. The progressively increasing number of new digital startups means regulations must be used to enforce certain safety and quality standards. Governments throughout the global community thus utilize their various agencies and some related global-level agencies to ensure that regulations and policies are strictly adhered to. Such agencies and government establishments ensure that all stakeholders adhere to the particular guidelines, regulations, and policies that address their particular sub-categories.
It’s a fact that governments around the world are now much more critical to the failure or success of digital health startups than ever before. Modern-day startups are playing a highly regulated, highly politicized, and traditionally government-run public space.
Thus, startups can’t afford to ignore government regulations and policies makers because:
- They need to actively engage policymakers so they can put heads together to find common grounds to all regulatory challenges that the industry might be facing.
- They can find ways to offer positive social changes for their native communities through brainstorming with the policymakers to determine where they fit into the regulatory and policy ecosystem.
- They can be transparent about their challenges thereby gaining the ability to approach all regulatory conflicts with real humility.
Any digital startup that fails to follow regulations, guidelines, or to implement government policies will hardly gain the three benefits mentioned above. Additionally, such a startup could face sanctions or even fail completely.
An example of a digital health startup that failed for ignoring regulations and policies is Theranos. The blood-technology startup had gone down for being somewhat secretive and was only revived after receiving additional funding of about $100 million in December 2017.
Getting the stakeholders to follow regulations can be challenging for the authorities. Therefore, authorities across the world use their agencies to enforce crucial aspects of regulations or compliance that include;
- Mobile Apps: Mobile digital health apps are replacing former inactive techniques. The mHealth industry was valued at $6.7 billion and is forecasted to reach $58.8 by 2020. Authorities must, therefore, ensure that all apps released into the market are fully compliant.
- Electronic Data Exchange: Security and privacy standards are two crucial principles in healthcare. All used health record systems must ensure that data is accessible and secure. Security standards that must be addressed to ensure that any digital health startup is compliant are technical, physical, and administrative security standards.
Keeping to these standards then ensures that medical records are safe, inter-operability of healthcare data is greatly enhanced, preserving the confidentiality of patients’ sensitive data, protection against cyber attacks on all types of medical devices, and so on.
Digital health policies in major eHealth markets
The world as a whole is doing a lot when it concerns developing policies and regulations meant to ensure the smooth operations of the digital health sector. In India, the EHR Standards/Model of India, National eHealth Authority (NEHA), Digital India Project, CIPAM, Innovative India Project, and ICMR are just a few of policy efforts from the authorities.
For the European region, efforts include the Task Force that was set up in February 2017 for boosting better digital health innovations, the European Connected Health Alliance, the European Interoperability Framework 2017, Digital Health Society consultation process, the Medical Device Regulation Journal 2017, the Medical Device Directive, and several others. All of the efforts are generally geared towards ensuring greater flexibility in using and collating data for digital health research and innovation across all EU member nations.
For the US, the FDA has truly been active in ensuring that digital health policies are adhered to by all stakeholders. Affordable Care Act, Medicaid, and Medicare, the 21st Century Cures Act, the FDA’s Digital Health Innovation Action Plan, and several other policies, regulations, and policy statement are meant to bring sanity and succour to the American digital health sector.
As for Israel, its digital health investments rose by 30% in 2016 alone. This is not unconnected with regulatory and policy programs and strategies that have enabled the country to offer unique capabilities in communication, cyber, information, and mobile technologies to the majority of its citizens. The nation’s health ministry operates a specialized department that is specifically dedicated to digital health. This department is connected to all other government departments to make the procurement of necessary resources east for it. This makes the implementation of government digital health policies very fast, convenient, and effective.
The above-mentioned policies and regulations covered virtually all areas of the digital health sector including eHealth, mHealth, telehealth, remote monitoring, clinical workflow, diagnostics, patient engagement, assistive devices, decision support, digital therapeutics, personal consultation devices, and so many more.
The policies guiding all these aspects of digital health are fully enforced in most nations of the world where they are fully in use, even though there is still room for improvement. And the different stakeholders of all global nations truly accept the regulations and the policies.
This is as they understand that only such policies can provide a level playground which is among the most important requirements for fast business growth and security.
Conclusion
The value and the rising popularity of the global digital health industry are just so massive that governments cannot afford to ignore it. The number of mHealth apps downloaded in 2016 was 3.2 billion, which is more than two times the 2013 downloads.
Such dramatic shifts obtained in the sector make it a lot more important that governments across the globe create regulations and policies to guard and further enhance the smooth running of the sector.
Thankfully, major global digital health markets like the US, Israel, India, Europe, and so on, have reacted favourably to these policies. This is the reason why the sector is seeing its largest chunk of investors’ funds yet. This also shows clearly that the digital health startups excitement caused by the dramatic rise of the industry can never afford to ignore policymakers if the industry is to advance than it already has.
As for the governments, the future is what they are all looking forward to. They have admitted via varying global platforms and conferences that they must be more liberal since the world has certainly turned into one small digital village.
Image credit: www.istockphoto.com