HumanOptics gets FDA approval for prosthetic iris
Custom-flex Artificial Iris can now be used to treat vision loss caused due to genetic disorders and damage to the eyes. It becomes the first prosthetic iris to receive FDA approval.
Human Eyes are complex organs. Each of the small parts works in co-ordination to present a clear vision. The major eye defects are associated with a problem in the iris (the coloured part), which controls the amount of light entering into the pupil(the central part).
The iris can be affected either due to any damage caused to the eye or due to a congenital defect called aniridia. The advancements in the health industry have enabled companies to create artificial iris, which can surgically replace the defective or the missing eye part.
HumanOptics, a German company has become the first one to receive the US Food and Drug Administration approval for its prosthetic iris product. CustomFlex Artificial Iris is now approved to be used in both adults and children.
It can give back vision to people who suffer from aniridia, a rare genetic disorder or other conditions such as albinism, traumatic injury or surgical removal due to melanoma.
People with such eye conditions have high sensitivity to light and face severe vision problems. Moreover, they look to get the iris replaced for cosmetic purposes.
Malvina Eydelman, M.D., director of the Division of Ophthalmic, and Ear, Nose and Throat Devices at the FDA’s Center for Devices and Radiological Health said, “Patients with iris defects may experience severe vision problems, as well as dissatisfaction with the appearance of their eye. Today’s approval of the first artificial iris provides a novel method to treat iris defects that reduce sensitivity to bright light and glare. It also improves the cosmetic appearance of the eye in patients with aniridia.”
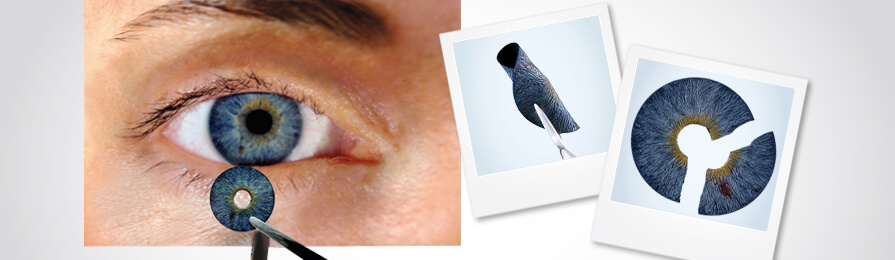
Made with medical-grade silicone, this artificial iris can be completely customised according to individual requirements; the size, as well as the colour, can be adjusted.
The prosthetic iris can be inserted by a surgeon after making a small cut in the eye. It can then be unfolded and smoothen with the help of suitable instruments. If required, sutures are done to hold the iris in place while normally, the anatomical eye structure is sufficient to do the task.
However, there are certain groups for which the custom-flex artificial iris is contraindicated. These include pregnant women, and people who suffer from uveitis, which is the severe chronic inflammation in the eye or, have microphthalmus, an abnormally small eye size, retinal detachment or chronic glaucoma that is untreated, cataract caused by rubella virus, abnormal blood vessels on the iris (rubeosis), damaged blood vessels or any other intraocular infections.
There are several other interesting technological advancements made to ensure better eye-health and vision to the masses. Recently, Simple Contacts, an Eyecare telemedicine startup raised $16M in funding.
Another example is the Swiss company Novartis that launched a mobile app to track eye-disease progression.
Image credit: www.humanoptics.com