Beta Bionic to conduct clinical testing of the AI-powered bionic pancreas for diabetes
The AI-powered bionic pancreas secures approval of the FDA to conduct clinical trials for fast acting insulin to cure diabetes Type-1.
Technology has advanced manifold in the past few years. Every industry is employing technology in its own way to get maximum benefit from it. A closed-loop system or artificial pancreas was one such device designed to monitor the blood glucose level of diabetes type-1 patients. This device monitors a person’s food intake and the rise in blood sugar levels and releases insulin automatically in accordance with it. When there is fall in blood sugar they also provide glucagon in its response. Now, there is a new optimization in this technology as well.
The combination of Artificial Intelligence (AI) and Continuous Glucose Monitoring (CGM) system by Dexcom targets towards constructing a bionic pancreas. The whole initiative is taken by a startup company Beta Bionics.
Following the approval from the FDA, the venture has started the home-use studies which are based on insulin-only configuration testing.
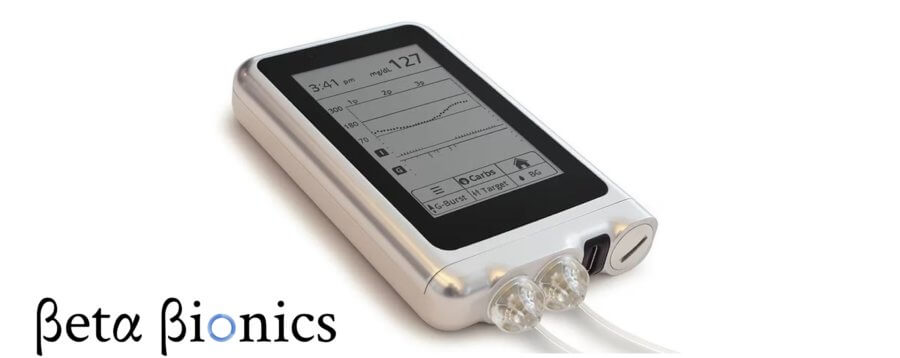
This will be the first trial to make use of the newly manufactured iLet device. The device carries the power of dosing two hormones: only glucagon, only insulin, or both of the hormones as required. It consists of a dual chambered, autonomous, infusion pump. The device actively resembles a biological pancreas for functionality. The innovative device can be easily worn on the body, which consists of a small cartridge containing the insulin.
The system comes with an AI component with clinically tested dosing algorithms. Its Machine Learning capacity enables it to give autonomously calculated dose of insulin or glucagon as required.
It continuously learns through the data from the monitor with the help of machine learning. The efficient technology adapts according to the patient’s need for insulin, adjusting to the individual’s blood glucose level.
The company is based in the US and the clinical study will be testing a fast-acting insulin called as “Fiasp”. The compound was developed by Novo Nordisk and has been recently approved. It will combine with the iLet bionic pancreas in adults suffering from diabetes type-1.
Another insulin “Lispro” and conventional insulin called as “Aspart” is also going to be functional in children and adults suffering from diabetes type-1. The first ever fast-acting insulin, Fiasp does not require any pre-meal recommendation for prescribed doses. It has the capability to get active in blood within 2.5 minutes.
The device is capable of controlling the patient’s blood sugar. It is functional as a bridge to cure diabetes Type-1 specifically.
But the company is hopeful about its application for diabetes Type-2 as well, as stated by the founder and CEO of the company, Ed Damiano.
The iLet bionic pancreas stands apart from an insulin pump devised by Dublin-based Medtronic. It works as an artificial pancreas and the company received the FDA approval back in 2016.
The company plans to ramp up the crucial trials in 2019 with the final design of iLet. The product launch is set to be in 2020, as reported by Globe News Wire.
The Global Chief Medical Officer of Novo Nordisk, Stephen Gough said that it is an innovation centered around patients specifically concentrating on the goal to defeat diabetes. The company is optimistic about the results of the clinical trial. It aims towards portraying the benefits of using the cutting-edge technology to battle diabetes.
Image credit: www.betabionics.org