Infobionic raises $50 Million to develop Arrhythmia detection platform
MoMeKardia, a cloud-based technology device will now facilitate more accurate and quick diagnosis of Arrhythmia.
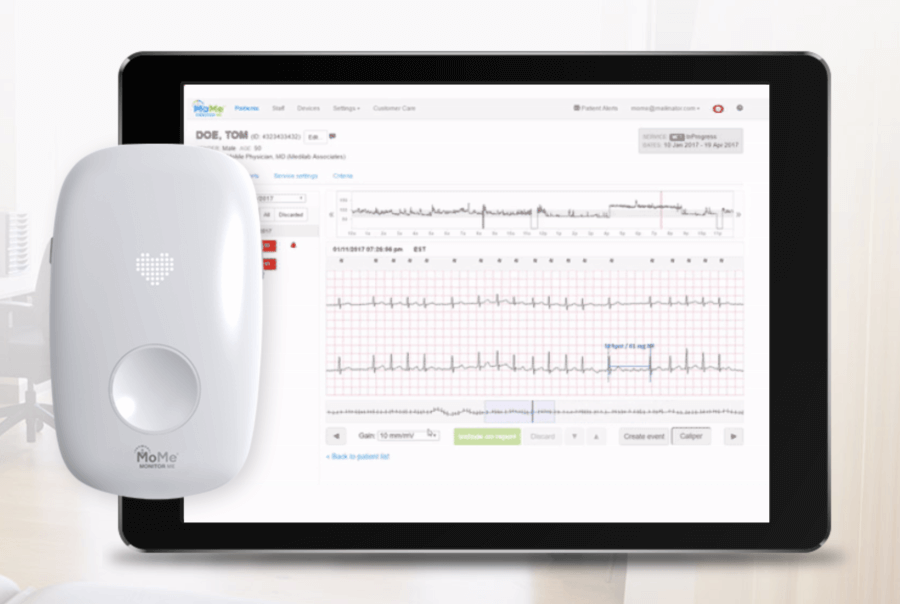
Infobionic is a Boston, United States of America based biotechnology and medical healthcare device development company. As a digital healthcare company, Infobionic has laid an initial focus on cardiac arrhythmia. Launched in 2011, Infobionic has successfully launched the MoMe system. The MoMe system is a compact remote like device that is capable of monitoring and detecting cardiac arrhythmias in patients. To receive a superior monitoring experience, patients are required to wear the device either around the neck or attach it to their belt.
The device is enabled with all patient information and effectively and efficiently improves patient clinical care continuously. Led by a team of experts in the field of medicine as well as technology, Infobionic has seamlessly merged cloud services with detailed arrhythmia detection to create, the MoMe Kardia.
The MoMe Kardia device platform influences cloud-based technology and analytics to provide real-time access to patient information and condition for diagnostic purposes.
MoMe Kardia is the first of its kind in the world. In anticipation of being a revolutionizing invention, Infobionic just raised 50 million dollars for the manufacturing of the product.
To review reports, users or healthcare experts can simply log onto a HIPPA– compliant portal that stores all the detected information. It also helps track medication and provides information regarding various cardiac related problems.
Since its inception in 2011, Infobionic has raised a total of approximately 75 million dollars in venture capital funding in all its financing rounds. The finances were raised by the following participants – Excel Venture Management, Eagle Investments, Safeguard Scientifics, Blue Cross and Blue Shield of Kansas, Inc and a subsidiary of the Blue Cross Blue Shield of Massachusetts – Zaffre Investments.
After being approved by the FDA in 2015 for being a ‘Hotler, Event and Mobile Cardiac Telemetry monitoring’ device, the company will work in collaboration with Biotronik. Infobionic signed a deal with Biotronik for exclusive distribution in order for the device to reach more consumers.
Since the previous year, MoMe Kardia’s monthly subscription has shot over 35% in growth rate. The market opportunities for a device like this is estimated to be a whopping 26.6 million dollars in the next 2 years.
With favourable conditions like the market and the collaborative distribution plans, Infobionic’s MoMe Kardia is sure to grow at a fast rate attracting a lot of users.
Infobionic’s MoMe Kardia is currently facing direct competition from products like iRhytm’s Zio Patch and an ECG device developed by Bardy Diagnostics.
It faces indirect competition from products that are sold over the counter like AliveCor’s Kardia band and smartwatches like the Apple Watch 4 that are capable of monitoring heart function.
The device can be really beneficial in the years to come provided proper funding and utilization of this innovative cloud-based technology is put to use.
Image credit: www.infobionic.com