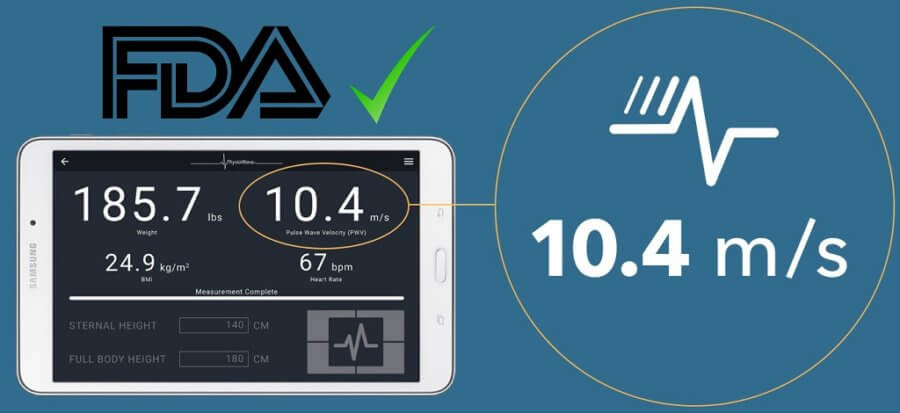
Physiowave gets FDA clearance for cardiovascular analyzer
Physio Wave Pro™ device is invented by Stanford University researchers which would measure Pulse Wave Velocity (PWV) along with pulse rate, weight, and BMI under one minute.
Small and large arteries are primarily responsible for cardiovascular disease and illnesses. Augmentation of the major arteries and arterial stiffness is a powerful precursor to potential health problems including renal complications, heart failure, sclerosis and even heart attacks. Systolic pressure, age, and are the most important factors in increasing Pulse Wave Velocity (PWV). During the ageing process, loss of elasticity and medical calcification in the arteries occurs.
As a result, measuring PWV is useful for studying the effects of, vascular diseases, ageing, vase-constricting agents on arteries and vase dilating.
Physiowave is a healthcare company that deals in providing Pulse Wave Velocity (PWV) solutions to heart patients. Group of Stanford researchers founded the company, as they believe, not many companies are working towards this issue because of the challenges associated with the problem. The researchers have worked hard to invent a way to measure PWV in a matter of a minute while giving patients, no extra visits to clinics.
PWV is a significant aspect of cardiovascular health. Considering the potential of PWV in determining cardiovascular disease, researchers have developed cardiovascular risk assessment biomarkers technology. The company has developed a product that is clinically valid home version.
The American Heart Association and American College of Cardiology, is supporting the measurement of Pulse Wave Velocity (PWV) after the research of 25 years. The researchers at AHA/ACC believed that PWV is worth in assessing arterial stiffness together with traditional risk factors to improve cardiovascular risk prediction.
Physiowave researchers developed PhysioWave Pro™ cardiovascular analyzer to identify the underlying problems in patient’s heart. The company announced that the product recently received FDA clearance.
Physio wave is the only scale-based PWV device on the market with Nokia’s recent withdrawal of the non-FDA-cleared PWV feature of its Body Cardio consumer scale. Greg Kovacs, Physio Wave’s founder and acting CEO, said, “We are thrilled to be bringing this new technology to clinical practices worldwide and look forward to partnering to develop a lower-cost version for consumers.”
He further mentioned that this measurement provides real, actionable information on cardiovascular health to everyone, and can work in concert with all manner of wearables and health apps. We believe we are at the beginning of a highly significant development in medicine.
PWV would certainly measure the stiffness of the blood vessels transporting blood from the heart to the body. Patients having increased stiffness are more prone to cardiovascular risks (e.g., coronary heart disease, stroke, and hypertension).
Physio Wave Pro™ device will analyze patients PWV, by allowing patients to stand on the device. The device, similar to a body weight scale, also measures pulse rate, weight, and BMI.
The analyses process would hardly take one minute, thus saving the time of doctors and patients. However, early deliveries to clinical customers are planned for later in 2018.
Image credit: www.physiowave.com