MC10 gets FDA approval for sensor platform for clinical trials
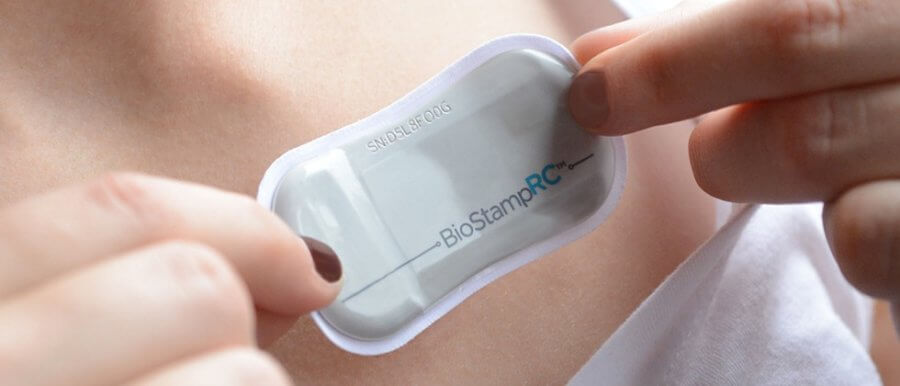
Cambridge, Massachusetts-based sensor maker MC10 ‘s BioStamp nPoint system got FDA 510(k) clearance. It can monitor direct data and derived data such as posture classification & heart rate variation.
The FDA, which is a well-known body in the US, deals with public health care where they ensure there is food safety; there are dietary supplements and supervision of tobacco products. dietary supplements and supervision of tobacco products. The FDA receives full support from the US government to enforce laws that govern the drug, federal food, and cosmetic industries. It also ensures that everything used in the hospitals meets the required standards.
Recently US healthcare company MC10 received its first FDA 510(k) clearance for the Bio Stamp nPoint system. Bio Stamp nPoint is a more unconventional version of the company’s non-FDA cleared BioStampRC. The Bio Stamp nPoint systems ensure that there are improvement and enhancement to BioStampRC.
FDA will regulate even medical devices that could endanger people’s life. Mechanical heart valve must be checked before they are released into the market. This is the reason, the BioStamp nPoint system is designed to meet both home and clinic needs and will be available for sale and rental beginning June this year.
The best thing about the system is that the sensor patches are reusable and can be trusted to monitor 24 hours at a time. The system has two apps- the patient-facing MC10 Link App and the researcher-facing MC10 Investigator app.
The patient can use MC10 Link app and get instructions on how to place the patches and even remind the patient to take medication. The researcher, on the other hand, uses the MC10 investigator app, where he will receive information about all the sensors. The system can be able to monitor the heart rate, posture and different things related to sleep.
In an interview with MobilehealthNews, MC10s’ senior vice president Don Fuchs said, “For new drug applications and for phase 1 through 4 FDA filings, the biometric sensors that would be used to collect data for those filings, and the pharma companies are looking for FDA-cleared products because they need to be able to point to a clinic validation.”
These biometric data collection platforms are to be used by professionals who work in the eHealth to collect physiological data that could be in the home and traditional clinics.
Dr.Arthur Combs who is the MD Chief Medical Officer of MC10 commented, “The nature, location, and endpoints of clinical trials for new drug development are all evolving rapidly. The Biostamp nPoint system was designed and clinically validated to meet this rapidly growing and expanding unmet need.”
Biostamp nPoint produces clinical quality medical grade physiological data from research subjects in their own homes, available to investigators from virtually anywhere.
The FDA will bring a great change in consumer health information by giving the public the protection they need. They are willing to go out and get marketers who claim that FDA to ensure everything in the market is good for human health has approved their products. The FDA reviews how effective and safe a product is before it goes out to the market.
Mc10’s Bio Stamp nPoint will surely improve the health care set up in the US. The company is planning to a partnership with pharmaceutical companies, thereby making innovations in the product. Bio Stamp nPoint will monitor not only direct data, but derived data including posture classification, heart rate variation, and a number of specific metrics related to sleep.
Image credit: www.mc10inc.com